Efficient ADHD Diagnosis System Based on Structural MRI and Specially Tailored Machine Learning Technique
Copyright ⓒ 2024 The Digital Contents Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-CommercialLicense(http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.

Abstract
In this study, an efficient attention deficit hyperactivity disorder (ADHD) diagnosis system based on structural MRI is proposed. This system leverages features extracted from structural MRI of seven brain regions—the Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus—available in the ADHD-200 database. These features, identified using region-of-interest (ROI) analysis, are employed to train an efficient classifier capable of distinguishing between three subtypes of ADHD: ADHD-C, ADHD-H, and ADHD-I, in addition to typically developing controls. For effective training, approximately 40,000 extracted ROI features were preprocessed by a specially tailored ROI feature selection method based on the genetic algorithm. This process selected a subset of the most significant ROI features. The chosen subset of ROI features was then used to train an efficient ADHD classifier employing the Extreme Learning Machine. Experimental results clearly demonstrate that the proposed approach outperforms existing techniques in terms of overall testing accuracy.
초록
본 논문은 Structural MRI를 기반으로 한 효율적인 주의력결핍 과잉행동장애(ADHD) 진단 시스템을 제시한다. 이 시스템은 Region-of-Interest(ROI) 접근 방식을 사용하여 ADHD-200 데이터베이스에 존재하는 7개 뇌 영역(Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus)의 Structural MRI에서 특징들을 추출한다. 추출된 특징들을 활용하여 ADHD를 세 가지 하위 유형(ADHD-C, ADHD-H, ADHD-I)와 TDC(Typical Development Controls)을 분류하기 위해 효율적인 분류기의 훈련을 시도한다. 효율적인 훈련을 위해 유전 알고리즘을 기반으로 특별히 맞춤화된 ROI 선택 방법(roiFSM)을 통해 ~40000개의 추출된 ROI 세트를 처리하고 가장 중요한 ROI의 하위 세트를 선택한다. 선택된 ROI 하위 집합은 Extreme Learning Machine을 사용하여 효율적인 ADHD 분류기를 훈련한다. 실험 결과는 제안된 본 접근 방식이 전반적인 테스트 정확도의 관점에서 기존 기술보다 우수한 성능을 지닌다는 점을 분명하게 보여준다.
Keywords:
Attention Deficit Hyperactivity Disorder, ADHD-200, MRI, Region-of-Interest Features Selection Method (roiFSM), Extreme Learning Machine (ELM)키워드:
주의력결핍 과잉행동장애, 자기공명영상, 관심 영역 특성 선택 방법 (roiFSM), 극한학습기계 (ELM)Ⅰ. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is neuropsychiatric disorder common among people of 7 to 21 years of age [1]. Recent studies specify three main types of ADHD: hyperactive (ADHD-H), abnormal impulsive (ADHD-I), and combined (ADHD-C). ADHD can be diagnosed by behavioral analysis and can be efficiently treated if ADHD diagnosis is made at an earlier stage. According to recent reviews, ADHD may have genetic [2], or environmental nature [3], but the origin of ADHD is not fully clear. Hence, new methods for ADHD diagnosis at an early stage must be developed, and may lead to a better understanding of the nature of ADHD.
Presently, ADHD diagnosis is usually based upon symptoms analysis [4]. Such methods are not always accurate and may be affected by human mistakes. Recent advances in medical imaging techniques, coupled with advanced machine learning allow a deep structural analysis of human brains affected by ADHD. Researchers use MRI (magnetic resonance imaging) [3], and SPECT (single-photon emission computed tomography) [5],[6]. These high precision medical imaging techniques highlight brain regions affected by ADHD and provide extra information for further analysis.
SPECT [6], or single-photon emission computed tomography, is an efficient medical imaging tool, which uses radioactive materials to highlight abnormal brain regions. MRI, or magnetic resonance imaging, gives high precision images of the human brain, and does not use harmful radioactive materials. MRI can be used multiple times without any health limitations, and allows research on dynamic brain processes. Recent deep analysis of the ADHD patients using MRI has highlighted abnormalities in various brain regions, such as the basal ganglia and frontostriatal regions [15], corpus callosum [13], amygdala and thalamus areas [9], cerebral volume [3], temporoparietal lobes [7], Cerebellar vermis [14], caudate [11], striatum [16], hippocampus [8],[12], and amygdala [10], among others.
ADHD symptoms include cognitive and emotional instabilities. Thus, the brain regions responsible for various emotions and cognitive processes associated with emotions may be affected by ADHD. The brain areas responsible for emotions and cognitive processes are: the Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus. Brain regions associated with human emotions were recently intensively studied in [8], [9], and [10]. The amygdala is responsible for accumulating emotions associated with fear [10]. The caudate is more complex and responsible for learning, short-term memory, planning of movements, motivations, and various emotions [11]. The hippocampus is responsible for the physiology of the brain as well as cognitive functions such as producing hormones, long-term memory, and various different kinds of responses [8],[12]. Research has already demonstrated the importance of the amygdala, caudate and hippocampus for human emotions and for ADHD research. According to recent studies, ADHD may also affect other brain regions. Thus, in this research we combine data from the Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus brain regions, and build an efficient ADHD classifier based on a specially tailored Region-of-Interest Feature Selection Method coupled with the Extreme Learning Machine. The proposed ADHD classifier shows higher classification accuracy compared to other state-of-the-art methods.
Classification of ADHD subtypes based on applying advanced machine learning to structural and functional MRI data has been widely studied recently. In [25] authors used a complex cognitive neuro-fuzzy interface for efficient classification of the 3 subtypes of ADHD (ADHD-C vs. ADHD-H vs. ADHD-I) and typically developing controls (TDC) taken from the ADHD-200 database. Later, in [27] authors used features extracted from Hippocampus and improved classification performance. In [20] authors picked the Amygdala and Cerebellar vermis to extract features and build an efficient ADHD classifier. In [28] authors reduced the number of samples from the ADHD-200 database and built an extreme learning machine (ELM) classifier with high accuracy. In [29] authors proposed using multi kernel learning (MLK) to build an efficient classifier with high accuracy. The famous deep learning technique was used in [31] and [32] for accurate ADHD classification.
In this paper we propose a complex analysis of seven brain regions from the ADHD-200 database (namely, the Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus) using a tailored region-of-interest Feature Selection Method (roiFSM). The ROI procedure extracts around 40000 features from the MRI of the seven examined brain regions available in the ADHD-200 database. The direct use of 40000 features to build an efficient classifier would be a big challenge due to redundancy and extreme complexity of the classification problem. Hence, a new method which efficiently deals with a large set of features to build an accurate classifier is needed. In this paper, we proposed a region-of-interest Feature Selection Method (roiFSM) - a smart feature reduction technique, which helps to build an efficient classifier for classification problems with a large feature space.
This paper is organized as follows: Section 1 is the introduction, in Section 2 the ADHD-200 database is discussed in detail, Section 3 introduces the proposed region-of-interest Feature Selection Method, experimental results are presented in Section 4, and Section 5 concludes the paper.
Ⅱ. ADHD-200 database
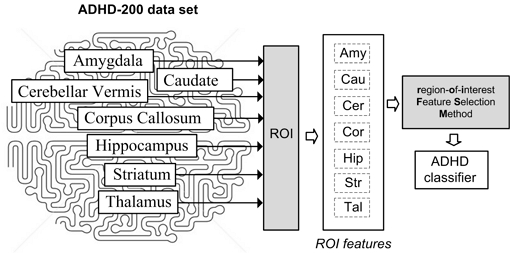
ADHD-200 [17] is the world’s biggest MRI database for ADHD research. ADHD-200 data was collected by many medical institutions all over the world, and includes MRI scans of 941 persons. ADHD-200 collects both structural and functional MRI. Among the 941 persons available in the ADHD-200 database, 581 are typical developed controls (TDC) or persons without ADHD, and the remaining 360 are ADHD patients: 137 with ADHD-I (inattentive ADHD), 13 with ADHD-H (hyperactive ADHD), and 210 with ADHD-C (combined inattentive and hyperactive ADHD). In this paper MRI data from seven brain regions (Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus) for all available 941 patients have been used for testing.
The original ADHD-200 database is divided into 770 training samples (487 TDC, 161 ADHD-C, 11 ADHD-H, and 111 ADHD-I: 290 females and 480 males) and 171 testing samples (94 TDC, 49 ADHD-C, 2 ADHD-H, and 26 ADHD-I: 65 females and 106 males).
1) ROI: region-of-interest
The Region-of-Interest ROI method is used to extract relevant features from MRI for further analysis. In this paper, the ROI was implemented following the Burner pipeline, which contains three main steps: the SMP or Statistical Parametric Mapping [21] step which separates gray matter and white matter, the data normalization step using DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) [22], and the transformation step in which each generated image is transformed into a space of population averages. The final step of the Burner pipeline generates Region-of-Interest masks using the Pickatlas tool [23].
In this paper, the Burner pipeline ROI (region-of-interest) method is used to extract a set of 41721 ROI features from the Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus. The Burner pipeline ROI (region-of-interest) method extracts 1050 ROI features from the Amygdala, 3904 ROI features from the Caudate, 6358 ROI features from the Cerebellar Vermis, 8536 ROI features from the Corpus Callosum, 6076 ROI features from the Hippocampus, 9359 ROI features from the Striatum, and 6438 ROI features from the Thalamus. The final set of 41721 ROI features is used in the proposed region-of-interest Feature Selection Method (roiFSM), which searches for a subset of best features for training of an accurate ADHD classifier using the Extreme Learning Machine.
III. Proposed Efficient ADHD Diagnostic Technique
The proposed region-of-interest Features Selection Method (roiFSM) is a key component in the proposed accurate ADHD diagnosis system based on structural MRI. The roiFSM reduces the unified set of 41721 features extracted from the seven examined brain regions (Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, Thalamus) and trains an accurate ADHD classifier using the Extreme Learning Machine. The framework of the proposed ADHD diagnosis system is presented in Fig. 1.
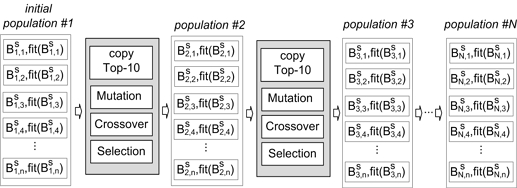
The proposed region-of-interest Feature Selection Method (roiFSM) has two major steps: a preprocessing step and a multiregional brain analysis step. In the preprocessing step, roiFSM deals with features extracted from each examined brain region separately, and the most relevant local ROI features for ADHD are selected. In the second multiregional brain analysis step roiFSM searches for relevant ROI features among all available 41721 ROI features extracted from the seven examined brain regions. In the final step, roiFSM produces a subset of ROI features from all 7 brain regions most relevant for ADHD. This selected set of features is used then to train an efficient ELM classifier. The detailed framework of the roiFSM is presented in Fig. 2. Detailed explanations about the preprocessing step and multiregional brain analysis step are given below.
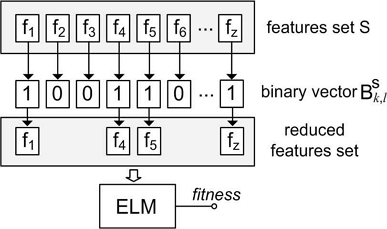
The proposed roiFSM is a modified version of the well-known Genetic Algorithm, adapted for ADHD data. roiFSM uses specially tailored Random Balanced Crossover and Mutation. In the framework presented in Fig. 2, roiFSM searches for subset of ROI features in the features set S, chosen from one of the seven examined brain regions (for example S = Amygdala, or S = Striatum, etc.) or a combination of all seven brain region together (S = Amygdala ∪ Caudate ∪ Cerebellar Vermis ∪ Corpus Callosum ∪ Hippocampus ∪ Striatum ∪ Thalamus).
The binary vector is a solution for roiFSM, which highlights chosen features by a logical “1”, and skipped features by a logical “0” (See example in Fig. 3.). Here S is the name of the features set, k is a population index, and l is the solution index in population k. Each population in roiFSM contains n solutions (n = 100). The initial population is built using binary vectors generated by allocating 100-200 logical “1” randomly in the vector. is a fitness function which uses the binary vector , creates a reduced set of features from set S, trains an ELM classifier, and uses the overall testing accuracy as a “fitness”. After collecting n fitness values the current population is updated by copying the Top-10% of the solutions to new generation, and generating the rest of the solutions using “Selection”, “Random Balanced Crossover” and “Random Balanced Mutation” as described below. For each newly generated solution the fitness function is processed, and fitness value is obtained. roiFSM updates population until a stopping criteria is met, which is no improvements in terms of fitness for the last 10 generations.
“Selection method” in the Genetic Algorithm selects one candidate from the current population based on its fitness value, such that solutions with higher fitness have higher chance to be picked. In this paper, the geometric ranking method [24] was used. The geometric ranking method defines the probability of selection for each solution based on the index of this solution in a set of sorted solutions. A detailed explanation of the geometric ranking method is given in [24].
“Random Balanced Crossover” is a key component of the proposed roiFSM. Crossover in the Genetic Algorithm exchanges genetic information between two solutions selected by “Selection method”, which mimics genetic crossover in nature. Crossover in nature is responsible for random gene exchange between two parents during reproduction and plays a critically important role for survival of a population.
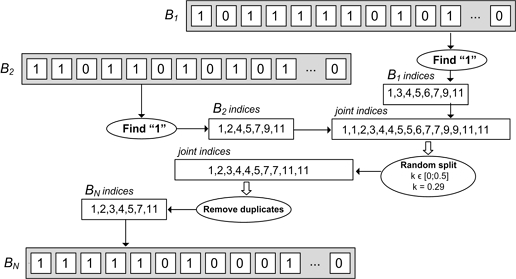
The Genetic Algorithm uses the adaptation of powerful crossover procedures from nature to solve various complex engineering problems, where analytical solutions are impossible to find. In the roiFSM the proposed “Random Balanced Crossover” creates a new solution by recombining logical “1”s extracted from two parents’ solutions, B1 and B2, selected by “Selection method” (see Fig. 4). In the first step, the “Random Balanced Crossover” extracts the indexes of logical “1”s from parent solutions B1 and B2. In the presented example (Fig. 4.) the set “B1 indices” is {1,2,4,5,7,9,11} and the set “B2 indices” is {1,3,4,5,6,7,9,11}. The set “joint indices” is {1,1,3,4,4,5,5,6,7,7,9,9,11,11}. The second step randomly splits the “joint indices” set with split coefficient k∈[0; 0.5]. The split coefficient is selected randomly each time crossover is lunched. In the presented example (see Fig. 4.) the split coefficient is k = 0.29, which means 29% of indices from “joint indices” must be discarded. The “Random Balanced Crossover” randomly choses 29% of indices from “joint indices” and discards them. The set of remaining indices is {1,3,4,4,5,7,7,9,11,11}. In the third step, the duplicated indices are discarded. The final “BN indices” set is {1,3,4,5,7,9,11}. Finally “Random Balanced Crossover” creates a new binary vector “BN using indices from ““BN indices”.
The proposed “Random Balanced Crossover” in the roiFSM framework has few significant advantages compared to other popular crossovers presented in literature. The “Random Balanced Crossover” modifies only logical “1”s from binary vectors B1 and B2, which always link to meaningfully selected features for use in training an ELM classifier. Random split in the “Random Balanced Crossover” gives good control over the number of logical “1”s in a new binary vector, “BN. The remove duplicates step coupled with the random split of duplicated indices means that duplicated indices have a higher chance to pass random split (indices 1,4,5,7,11 in Fig. 4.) and lower chance to be discarded (index 9). This combination of benefits of the “Random Balanced Crossover” significantly speeds up the searching process of the roiFSM and helps to find an optimal solution in reasonable time.
“Mutation” is another critical component of the Genetic Algorithm, which mimics genetic mutations in nature. Mutation in nature gives species the possibility to acquire novel characteristics, which may/or may not produce significant improvement. Such improvements may be impossible to achieve by crossover. Only the combination of crossover and mutation enables survival in nature. The mutation operator in the Genetic Algorithm generates a solution with randomly modified genes. roiFSM uses Random Balanced Mutation which generates a new solution with 50 – 200 logical "1”s allocated randomly.
roiFSM preprocessing step: In the preprocessing step roiFSM searchs for the optimal subset of features in each brain region separately. roiFSM creates 7 sets of best solutions with highest fitness corresponding to each brain region: Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus. The roiFSM crossover probability is 80% and mutation probability is 20% in each population. The population size is n = 100, and the stopping criteria is no fitness improvement for the last 10 populations.
roiFSM multiregional brain analysis step: In the multiregional brain analysis step features from all seven examined brain regions are used to build a unified set of 41721 ROI features. To speed up the searching process of the Genetic Algorithm the best solutions from preprocessing step are transferred to the roiFSM: multiregional brain analysis step. The Genetic Algorithm settings for roiFSM: multiregional brain analysis step are: crossover probability 80% and mutation probability 20%, population size n = 500, and stopping criteria is no fitness improvement for the last 50 populations.
IV. Extreme Learning Machine
Extreme Learning Machine is the machine learning tool which is used to train an ADHD classifier for 4 classes: 3 subtypes of ADHD (ADHD-I, ADHD-H and ADHD-C) and typical developed controls TDC. The ELM is a classical one hidden layer feed-forward neural network with Gaussian neurons where input weights are assigned randomly, and output weights are calculated analytically [18]. The bias of the hidden neurons is assigned randomly. For more details about ELM refer to [18].
V. Experimental Results
The proposed roiFSM was tested with all available data from the ADHD-200 database [17]. 941 patients were efficiently classified and the experimental results are provided in this Section.
The roiFSM: multiregional brain analysis step defines the best set of 1328 features from all seven brain regions: Amygdala (123 features), Caudate (98), Cerebellar Vermis (234), Corpus Callosum (265), Hippocampus (196), Striatum (201), and Thalamus (211). The selected set of ROI features was used to train an ELM classifier with overall training accuracy of 69.57% and overall testing accuracy of 67.06%.
The concept of confusion matrices can be used to display the classification performance of the best ADHD classifier created by roiFSM. The confusion matrix displays a distribution of the correct and incorrect classifications in respect of the class label. For the proposed ADHD classification problem, the size of the confusion matrix is 4 by 4, where 4 is the number of classes. In the confusion matrix rows link to the actual class label, and columns link to the predicted class label. If the ADHD classifier predicts the class correctly, the predicted class label matches the actual label and the corresponding cell in the confusion matrix is updated. In the confusion matrix correctly classified samples are allocated along the main diagonal, and incorrectly classified samples are allocated in other matrix cells. Confusion matrices for training and testing experiments of the best ADHD classifier found is presented in Tables 1 and 2.
For example, in Table 1 the first row displays distribution of the correct and incorrect samples classifications of class 1 (TDC). The first number is 286, this number is located in the main diagonal, so 286 is the number of correctly classified TDC samples among 486. The second number (112) in the first row represents TDC samples (actual class label is 1) which were classified as ADHD-C (predicted class label is 2). The third number (7) in the first row represents TDC samples (actual class label is 1) which were classified as ADHD-H (predicted class label is 3). The fourth number (81) in the first row represents TDC samples (actual class label is 1) which were classified as ADHD-H (predicted class label is 4). Similarly, sample distributions for ADHD-C, ADHD-H, and ADHD-I are presented in the second, third and fourth rows of the confusion matrix.
The proposed roiFSM was compared with various automatic ADHD diagnosis techniques. Many ADHD classifiers presented in literature focus on the binary classification problem [19], i.e., ADHD vs TDC. Such binary classification problem is much simpler compared to the 4-class problem examined here, and binary classifiers therefore should be expected to show a relatively higher accuracy. The more complex 3 or 4 class ADHD classification problems can be simplified by reducing the number of available samples, which makes problems easier to solve and leads to higher classification accuracy. The examined four-class classification problem is much harder in nature and needs more sophisticated machine learning tools.
The four-class ADHD classification problem was solved in [25] by using a Meta-Cognitive Neuro-Fuzzy Interface System; authors reported a testing accuracy of around 56%. In [27] a feature selection approach helped to improve the accuracy to 58,6%. Both methods used features extracted from Hippocampus brain reg ion only. In [26] authors used features extracted from the visual cortex brain regions, cingulate cortex, prefrontal cortex and reduced set of samples, and achieved testing accuracy of around 35.19%.
Among ADHD subtypes, ADHD-H has far fewer samples in the database than ADHD-I and ADHD-C, which makes the ADHD subclasses extremely unbalanced. The ADHD classification problem can be significantly simplified by removing the ADHD-H class. The three-class classification problem thus obtained, i.e., TDC vs. ADHD-I vs. ADHD-I is well researched. In [28] the three-class ADHD classification problem was solved for a reduced number of samples. Authors achieved testing accuracy of about 60.78%. In [30] the Multi-Region Risk-Sensitive Cognitive Ensembler solved the same three-class classification problem for all available ADHD-200 samples and achieved a testing accuracy of about 86%.
Among all methods cited above, only the methods presented in [25] and [27] solved the four-class ADHD classification problem using all available ADHD-200 samples (770 training samples and 171 testing samples). Thus, fair comparison of the method in this paper is only possible with the methods of [25] and [27]. The proposed roiFSM outperforms these methods by 10.98 % and 8.38% respectively, in terms of overall testing accuracy.
VI. Conclusion
The proposed region-of-interest Features Selection Method efficiently classifies three subtypes of ADHD (ADHD-C, ADHD-I, ADHD-H) and TDC. The roiFSM searches for the most significant ROI features in seven brain regions: Amygdala, Caudate, Cerebellar Vermis, Corpus Callosum, Hippocampus, Striatum, and Thalamus. Finally, the roiFSM defines an optimal subset of 1328 ROI features from all seven brain regions: Amygdala (123 features), Caudate (98), Cerebellar Vermis (234), Corpus Callosum (265), Hippocampus (196), Striatum (201), Thalamus (211). The selected set of ROI features was used to train an ELM classifier with overall training accuracy 69.57% and overall testing accuracy 67.06%. Experiments clearly indicate the advantage of the proposed roiFSM in comparison to existing techniques presented in the literature.
The proposed region-of-interest Features Selection Method can be improved further by using a more advanced framework of the Feature Selection Method and by using more efficient crossover and mutation.
Acknowledgments
The authors would like to thank the ADHD-200 consortium and the Neuro Bureau for making the MRI data available. We also thank the ADHD-200 Pre-processed initiative and Dr. Carlton Chu for the Burner pipeline. This work is supported by The Catholic University of Korea, Research Fund 2020.
References
- American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), 4th ed. Washington, DC: Author, 2000.
-
T. Banaschewski, K. Becker, S. Scherag, B. Franke, and D. Coghill, “Molecular Genetics of Attention-Deficit/Hyperactivity Disorder: An Overview,” European Child & Adolescent Psychiatry, Vol. 19, No. 3, pp. 237-257, March 2010.
[https://doi.org/10.1007/s00787-010-0090-z]

-
M. V. Cherkasova and L. Hechtman, “Neuroimaging in Attention-Deficit Hyperactivity Disorder: Beyond the Frontostriatal Circuitry,” The Canadian Journal of Psychiatry, No. 54, No. 10, pp. 651-664, October 2009.
[https://doi.org/10.1177/070674370905401002]

-
V. Vuontela, S. Carlson, A.-M. Troberg, T. Fontell, P. Simola, S. Saarinen, and E. T. Aronen, “Working Memory, Attention, Inhibition, and Their Relation to Adaptive Functioning and Behavioral/Emotional Symptoms in School-Aged Children,” Child Psychiatry & Human Development, Vol. 44, No. 1, pp. 105-122, February 2013.
[https://doi.org/10.1007/s10578-012-0313-2]

-
D. G. Amen, T. A. Henderson, and A. Newberg, “SPECT Functional Neuroimaging Distinguishes Adult Attention Deficit Hyperactivity Disorder from Healthy Controls in Big Data Imaging Cohorts,” Frontiers in Psychiatry, Vol. 12, 725788, November 2021.
[https://doi.org/10.3389/fpsyt.2021.725788]

-
H. Schneider, J. F. Thornton, M. A. Freeman, M. K. McLean, M. J. van Lierop, and J. Schneider, “Conventional SPECT Versus 3D Thresholded SPECT Imaging in the Diagnosis of ADHD: A Retrospective Study,” The Journal of Neuropsychiatry, Vol. 26, No. 4, pp. 335-343, 2014.
[https://doi.org/10.1176/appi.neuropsych.12110280]

-
J. N. Giedd and J. L. Rapoport, “Structural MRI of Pediatric Brain Development: What Have We Learned and Where Are We Going?,” Neuron, Vol. 67, No. 5, pp. 728-734, September 2010.
[https://doi.org/10.1016/j.neuron.2010.08.040]

-
H. Suzuki, K. N. Botteron, J. L. Luby, A. C. Belden, M. S. Gaffrey, C. M. Babb, ... and D. M. Barch, “Structural-Functional Correlations between Hippocampal Volume and Cortico-Limbic Emotional Responses in Depressed Children,” Cognitive, Affective, & Behavioral Neuroscience, Vol. 13, No. 1, pp. 135-151, March 2013.
[https://doi.org/10.3758/s13415-012-0121-y]

-
I. Ivanov, R. Bansal, X. Hao, H. Zhu, C. Kellendonk, L. Miller, ... and B. S. Peterson, “Morphological Abnormalities of the Thalamus in Youths with Attention Deficit Hyperactivity Disorder,” The Americal Journal of Psychiatry, Vol. 167, No. 4, pp. 397-408, April 2010.
[https://doi.org/10.1176/appi.ajp.2009.09030398]

-
K. J. Ressler, “Amygdala Activity, Fear, and Anxiety: Modulation by Stress,” Biological Psychiatry, Vol. 67, No. 12, pp. 1117-1119, June 2010.
[https://doi.org/10.1016/j.biopsych.2010.04.027]

-
C. A. Seger and C. M. Cincotta, “The Roles of the Caudate Nucleus in Human Classification Learning,” Journal of Neuroscience, Vol. 25, No. 11, pp. 2941-2951, March 2015.
[https://doi.org/10.1523/JNEUROSCI.3401-04.2005]

-
K. S. Anand and V. Dhikav, “Hippocampus in Health and Disease: An Overview,” Annals of Indian Academy of Neurology, Vol. 15, No. 4, pp. 239-246, October-December 2012.
[https://doi.org/10.4103/0972-2327.104323]

-
A. M. H. Onnink, M. P. Zwiers, M. Hoogman, J. C. Mostert, C. C. Kan, J. Buitelaar, and B. Franke, “Brain Alterations in Adult ADHD: Effects of Gender, Treatment and Comorbid Depression,” European Neuropsychopharmacology, Vol. 24, No. 3, pp. 397-409, March 2014.
[https://doi.org/10.1016/j.euroneuro.2013.11.011]

-
K. A. Coffman, R. P. Dum, and P. L. Strick, “Cerebellar Vermis is a Target of Projections from the Motor Areas in the Cerebral Cortex,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 108, No. 38, pp. 16068-16073, September 2011.
[https://doi.org/10.1073/pnas.1107904108]

- E. Perlov, A. Philipsen, L. T. van Elst, D. Ebert, J. Henning, S. Maier, ... and B. Hesslinger, “Hippocampus and Amygdala Morphology in Adults with Attention-Deficit Hyperactivity Disorder,” Journal of Psychiatry & Neuroscience, Vol. 33, No. 6, pp. 509-515, November 2008.
-
B. S. Kim and H.-I. Im, “The Role of the Dorsal Striatum in Choice Impulsivity,” Annals of the New York Academy of Sciences, Vol. 1451, No. 1, pp. 92-111, September 2019.
[https://doi.org/10.1111/nyas.13961]

-
The ADHD-200 Consortium, “The ADHD-200 Consortium: A Model to Advance the Translational Potential of Neuroimaging in Clinical Neuroscience,” Frontiers in Systems Neuroscience, Vol. 6, 62, September 2012.
[https://doi.org/10.3389/fnsys.2012.00062]

-
G.-B. Huang, Q.-Y. Zhu, and C.-K. Siew, “Extreme Learning Machine: Theory and Applications,” Neurocomputing, Vol. 70, No. 1-3, pp. 489-501, December 2006.
[https://doi.org/10.1016/j.neucom.2005.12.126]

-
B. S. Mahanand, R. Savitha, and S. Suresh, “Computer Aided Diagnosis of ADHD Using Brain Magnetic Resonance Images,” in Proceedings of the 26th Australian Joint Conference on Advances in Artificial Intelligence (AI 2013), Dunedin, New Zealand, pp. 386-395, December 2013.
[https://doi.org/10.1007/978-3-319-03680-9_39]

-
B. Rangarajan, S. Suresh, and B. S. Mahanand, “Identification of Potential Biomarkers in the Hippocampus Region for the Diagnosis of ADHD Using PBL-McRBFN Approach,” in Proceedings of the 13th International Conference on Control Automation Robotics & Vision (ICARCV), Singapore, pp. 17-22, December 2014.
[https://doi.org/10.1109/ICARCV.2014.7064272]

- K. J. Friston, J. T. Ashburner, S. J. Kiebel, T. E. Nichols, and W. D. Penny, Statistical Parametric Mapping: The Analysis of Functional Brain Images, London, UK: Academic Press, 2007.
-
J. Ashburner, “A Fast Diffeomorphic Image Registration Algorithm,” NeuroImage, Vol. 38, No. 1, pp. 95-113, October 2007.
[https://doi.org/10.1016/j.neuroimage.2007.07.007]

-
J. A. Maldjian, P. J. Laurienti, R. A. Kraft, and J. H. Burdette, “An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-Based Interrogation of fMRI Data Sets,” NeuroImage, Vol. 19, No. 3, pp. 1233-1239, July 2003.
[https://doi.org/10.1016/S1053-8119(03)00169-1]

-
S. Suresh, S. N. Omkar, V. Mani, and T. N. Guru Prakash, “Lift Coefficient Prediction at High Angle of Attack Using Recurrent Neural Network,” Aerospace Science and Technology, Vol. 7, No. 8, pp. 595-602, December 2003.
[https://doi.org/10.1016/S1270-9638(03)00053-1]

-
V. Sachnev, “An Efficient Classification Scheme for ADHD Problem Based on Binary Coded Genetic Algorithm and McFIS,” in Proceedings of 2015 International Conference on Cognitive Computing and Information Processing (CCIP), Noida, India, pp. 1-6, March 2015.
[https://doi.org/10.1109/CCIP.2015.7100690]

-
D. Kuang, X. Guo, X. An, Y. Zhao, and L. He, “Discrimination of ADHD Based on fMRI Data with Deep Belief Network,” in Proceedings of the 10th International Conference on Intelligent Computing in Bioinformatics, Taiyuan, China, pp. 225-232, August 2014.
[https://doi.org/10.1007/978-3-319-09330-7_27]

-
V. Sachnev and S. Suresh, “An ADHD Diagnostic Approach Based on Binary-Coded Genetic Algorithm and Extreme Learning Machine,” Journal of Computing Science and Engineering, Vol. 10, No. 4, pp. 111-117, December 2016.
[https://doi.org/10.5626/JCSE.2016.10.4.111]

-
M. N. I. Qureshi, B. Min, H. J. Jo, and B. Lee, “Multiclass Classification for the Differential Diagnosis on the ADHD Subtypes Using Recursive Feature Elimination and Hierarchical Extreme Learning Machine: Structural MRI Study,” PLoS ONE, Vol. 11, No. 8, e0160697, August 2016.
[https://doi.org/10.1371/journal.pone.0160697]

-
D. Dai, J. Wang, J. Hua, and H. He, “Classification of ADHD Children through Multimodal Magnetic Resonance Imaging,” Frontiers in Systems Neuroscience, Vol. 6, 63, September 2012.
[https://doi.org/10.3389/fnsys.2012.00063]

-
V. Sachnev, S. Suresh, N. Sundararajan, B. S. Mahanand, M. W. Azeem, and S. Saraswathi, “Multi-Region Risk-Sensitive Cognitive Ensembler for Accurate Detection of Attention-Deficit/Hyperactivity Disorder,” Cognitive Computation, Vol. 11, No. 4, pp. 545-559, August 2019.
[https://doi.org/10.1007/s12559-019-09636-0]

-
Y. Chen, Y. Tang, C. Wang, X. Liu, L. Zhao, and Z. Wang, “ADHD Classification by Dual Subspace Learning Using Resting-State Functional Connectivity,” Artificial Intelligence in Medicine, Vol. 103, 101786, March 2020.
[https://doi.org/10.1016/j.artmed.2019.101786]

-
H. Ke, D. Chen, Q. Yao, Y. Tang, J. Wu, J. Monaghan, ... and D. McAlpine, “Deep Factor Learning for Accurate Brain Neuroimaging Data Analysis on Discrimination for Structural MRI and Functional MRI,” IEEE/ACM Transactions on Computational Biology and Bioinformatics, March 2023.
[https://doi.org/10.1109/TCBB.2023.3252577]

저자소개

2004년:Komsomolsk-na-Amure State Technical University, M.S.
2009년:Korea University, Center of Information Security and Technology (CIST), PhD
2010년~현 재: Catholic University of Korea, Associate Professor.
His research interests include machine learning, bioinformatics, digital watermarking, steganography, and image processing.

2003년:M.S, Visvesvaraya Technological University, Belagavi, India
2012년:PhD, Visvesvaraya Technological University, Belagavi, India, M.Tech
2003년~현 재: Sri Jayachamarajendra College of Engineering (SJCE), JSS Science and Technology University, Mysuru.
Dr. Mahanand has published more than 50 research articles in various peer-reviewed journals and reputed conferences. His research interests are in the areas of artificial intelligence, machine learning, and medical image analysis.